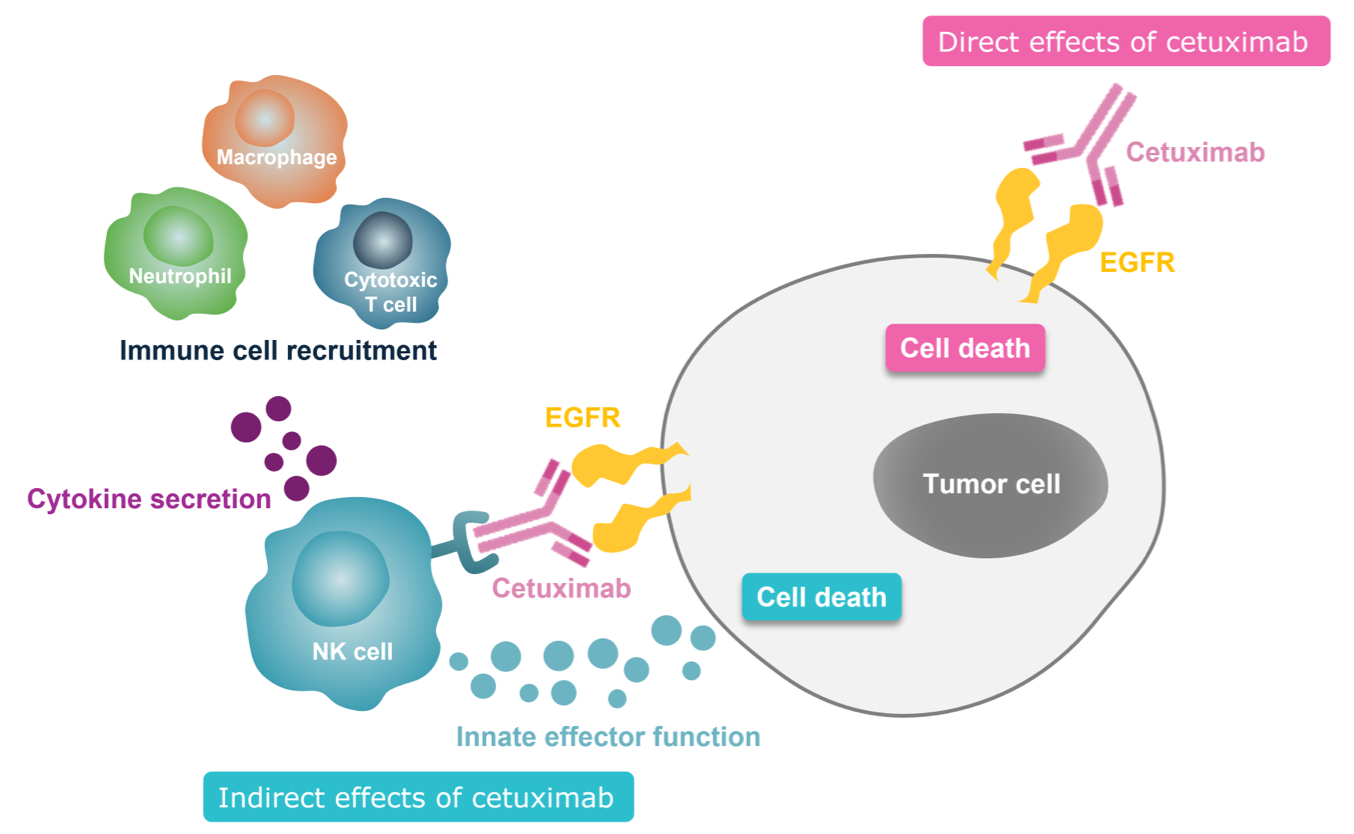
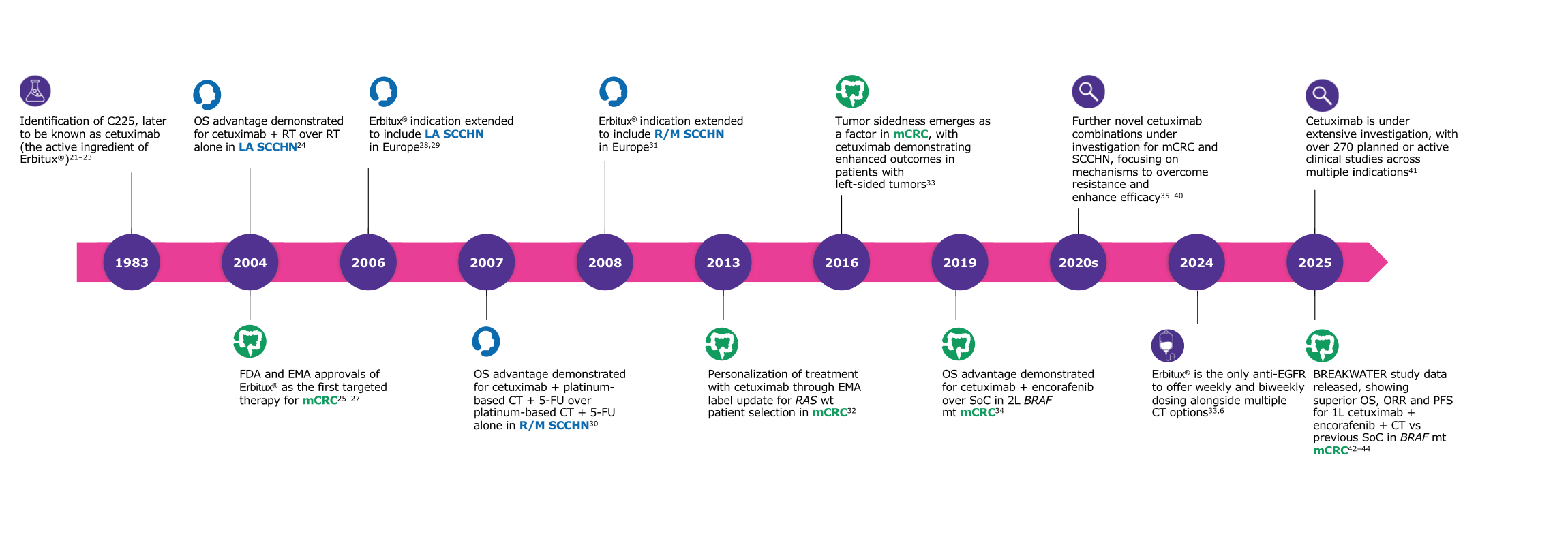
1L, first-line; ADCC, antibody-dependent cell-mediated cytotoxicity; ASCO, American Society of Clinical Oncology; CDC, complement-dependent cytotoxicity; CRC, colorectal cancer; ESMO, European Society for Medical Oncology; FDA, Food & Drug Administration; HPV, human papillomavirus-positive; ICD, immunogenic cell death; LA SCCHN, locally advanced squamous cell carcinoma of the head and/or neck; MoA, mechanism of action; mCRC, metastatic colorectal cancer; OPC, oropharyngeal cancer; R/M, recurrent and/or metastatic; SCCHN, squamous cell carcinoma of the head and/or neck; SoC, standard of care; VEGF, vascular endothelial growth factor.
 Global incidence and burden
Global incidence and burden Geographical variation
Geographical variation